

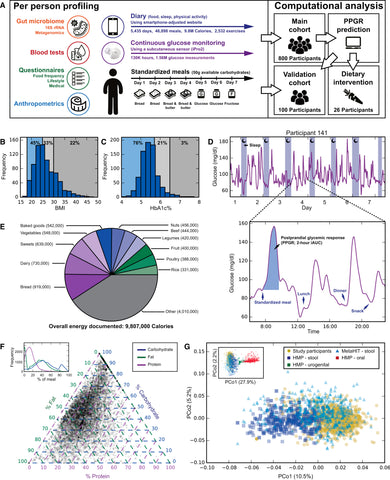
Many chronic diseases start with a slow progression of metabolic dysfunction that can be tied to the overconsumption of sugar. One of the diseases with documented connection to glucose metabolism and metabolic dysregulation is Parkinson’s disease. People with Parkinson’s disease should be mindful of their intake of simple carbohydrates and sugar, found in things like baked goods, packaged foods, cereals, etc. Studies suggest that having persistent high blood sugar, insulin resistance or type 2 diabetes, may accelerate disease progression. (source 1,source 2)

How Gut Bacteria Plays a Role
Research is also connecting the impact of various sugars on the microbiome and the potential impact on memory and cognition (source). It turns out that the microbiota in your gut is a key determinant of how readily sugar enters your blood after eating. (source) Therefore, having the right bacteria in your gut can balance and improve your blood sugar levels.
Insulin Resistance in People with Parkinson’s
In 2018, researchers from Cedar-Sinai Medical Center published a study in the Journal of Parkinson's disease, titled "High Prevalence of Undiagnosed Insulin Resistance in Non-Diabetic Subjects with Parkinson's disease" (Hogg et al, 2018). The study revealed that nearly 60% of the non-diabetic study participants had undiagnosed insulin resistance - despite having normal blood sugar levels. Insulin resistance was connected to Body Mass Index in the study, but the sample was too small to determine a correlation between insulin resistance and cognitive impairment.
A data analytics project I worked on about a decade ago provided access to a large set of hospital data, including over 40,000 Parkinson's patients over a 15-year period. During the project we looked at de-identified Parkinson's patient data based on billing codes. Using these codes we were able to look for additional health issues (comorbidities) in the patients with a PD diagnosis. The two most prevalent comorbidities in Parkinson's were diabetes and cardiovascular disease. Sugar consumption and insulin resistance are well documented contributors to cardiovascular disease and diabetes.
Why don’t doctors notice these connections?
Our current approach to science and medicine is very specialized. As a result, the connections across diseases are often missed. Neurologists don't look at blood chemistry routinely, so it's likely a neurologist would have no idea of this potential connection between diabetes, insulin resistance and Parkinson's.
(source)
Cardiologists are focused on the heart and don’t look for neurological issues other than cardiac related neuropathy. Our system ignores the fact that all of these diseases begin with metabolic disease. Something in the system isn’t functioning properly and that often goes back to the metabolism of sugars in the microbiome.
Should People with Parkinson’s Avoid Sugar?
Based on my many conversations with people with Parkinson's and their partners, I noticed a tendency toward sugar addiction. I often comment on this when I attend Parkinson's support group meetings or even research presentations at Universities where the tables are spread with donuts and candy - the very "foods" you should NOT be eating.
People often confide in me that they have a “sweet tooth.” This is certainly the case with my husband, John. He loves sweets and carbs and always has. He was a long distance runner and the conventional wisdom was all about carbohydrate loading before a long run or a competitive race. Over time this carb loading impacts the body and sets up the landscape to trigger insulin resistance.
To understand the connection between sugar and Parkinson’s, it can be helpful to start with insulin and how it functions in the body.

So what is insulin?
- Insulin is an anabolic (muscle building) hormone secreted by b cells in the pancreas that regulates glucose homeostasis in cells major ones being liver, skeletal muscle and fat cells
- Insulin signals the cells in the body to absorb the sugar from your blood where it is used for energy or in the case of fat, stored for later use to produce energy
- Insulin controls switch between fasting (use of energy stores) and feeding (storage of sugars - glucose/fructose in fat)
- Insulin is now known to have a much broader role in the body
- Insulin-like signaling regulates processes such as reproduction and lifespan
- Insulin signalling is involved in brain plasticity, learning and memory
- Insulin can modulate the expression of neuropeptides involved in the regulation of food intake within the central nervous system
What is insulin resistance?
Insulin resistance occurs when your cells become less sensitive to the insulin and receptors on the cells can no longer pull glucose in to the cell for energy. Conventional wisdom says that this insensitivity then requires more and more insulin to get the same action. Dr Jason Fung has proposed an alternative understanding of insulin resistance as the over production of insulin. He describes it in this way: Excess food consumption, especially carbohydrates, results in more and more insulin secretion to drive blood sugar into cells. Insulin is a fat storage hormone which blocks the burning of fat and converts any excess sugars into fat for stored energy. The more carbs you eat, the more likely you are to have chronically high insulin levels and a gradual build of fat..You can think of it in the same way that an alcoholic takes more and more alcohol to feel the same effect over time.
Something I learned while writing this article is how certain medications for Parkinson’s can cause insulin resistance. One of the most commonly prescribed drugs for Parkinson’s, Levodopa-Carbidopa is known to increase insulin resistance over time (reference). This increases the likelihood of insulin resistance in people with Parkinson’s.
What can happen when you become insulin resistant?
- Excess sugar can build up in the blood, damaging the blood vessels to your organs
- It can develop into diabetes with many side effects such as neuropathy, vision problems and cardiovascular issues
- Contributes to increased systemic inflammation
- Puts strain on the pancreas where insulin in made and is correlated with pancreatic cancer

(source)
What are potential causes or contributing factors to insulin resistance?
- Poor diet is the number one contributor
- Diets high in processed foods
- Vegetable and seed oil consumption
- added sugars, especially high fructose corn syrup
- high carbohydrate diets - white rice, white potatoes, highly processed white flour
- Inflammation in the body from other causes, eg. infection, contributing to a vicious cycle
- Inactivity, reduced physical exercise
- Being overweight or obese or a state known as lean obesity, where fat is localized in the belly but the rest of the body is thin
- Chronic stress may also contribute to insulin resistance as stress hormones interfere with insulin signals
- Medications
- Fluorquinolone antibiotics
- Beta blockers for blood pressure
- Anti-psychotics
- Immunosuppressants
- Glucocorticoids
- PD medication Levodopa-Carbidopa
How does insulin resistance play a role in PD?
There are an increasing number of studies suggesting that insulin resistance impacts dopamine function in the brain and muscles.
A study from researchers at Harvard Medical School showed that insulin resistance increased dopamine turnover in the dopaminergic neurons in the brain. Both Type1 and Type 2 diabetes are associated with age-related cognitive impairment. The Harvard study in neurons showed that changes in key molecular pathways were the consequence of loss of insulin signalling. Stress is a known contributor to Parkinson’s disease and stress hormones like cortisol and adrenaline can interfere with insulin signalling. Insulin resistance in the brain induces mitochondrial dysfunction and impacts dopamine in the brain.
This is particularly interesting given the data indicating that Levodopa-Carbodopa causes insulin resistance based upon this research in muscle tissue and animal model (reference). Glucose transport in to the muscles is stimulated by insulin in skeletal muscles. Skeletal muscle is the predominant insulin response tissue. This study from 2004, showed that levodopa-carbidopa decrease insulin-stimulated glucose transport, glycogen accumulation (glucose stored in fat) and glycogen synthase activity in skeletal muscle. This provides some insight to the descriptions from Parkinson’s patients about how their legs feel when they are not working properly. Lack of glucose in the muscles would, of course, make the legs feel heavy and unresponsive.
Separate research has shown that MAO inhibitors, drugs often used in the treatment of Parkinson's, can impact glucose levels in the blood, indicating that these drugs can improve glucose tolerance and insulin sensitivity.
Our relationship with sugar is complicated.
Our brains depend on glucose as the main source of energy and tight regulation of glucose metabolism is critical for brain physiology. Disrupted glucose homeostasis affects cell death pathways and forms the basis for many brain disorders. Glycogen storage is the primary mechanism for managing glucose homeostasis in the body. 1% of glycogen is stored in the muscles, with the rest stored in the liver. Glycogen stored in the liver is converted back in to glucose when needed to maintain glucose homeostasis. Given the potential impact on glycogen storage from the primary Parkinson’s medication, it is more likely that people with Parkinson’s will have trouble maintaining glucose homeostasis. The Texas Institute for Neurological Disorders reports that too much sugar/glucose can lead to memory deficits and a high sugar diet can lead to dopamine deficits as the brain becomes used to high levels of sugar. The brain can use glucose, lactate or ketones for energy. Recent research has shown that ketones are some of the best brain fuel.
Ketogenic Diet for Parkinson’s Patients?
Research is finding that the best way to reduce insulin resistance is through a diet of healthy fats, low carbs and intermittent fasting.
A 2018 study compared a low fat vs a ketogenic diet in Parkinson’s. In the ketogenic diet, 80% of the calories were derived from healthy fats. Healthy fats include fats like olive oil, coconut oil, and fats from oily fish like sardines and salmon. Results showed that the ketogenic diet not only improved motor symptoms but it also improved non-motor symptoms like urinary problems, tiredness, cognitive issues and daytime sleepiness. The ketogenic diet is one of the main approaches today for addressing insulin resistance and resetting metabolism. Sugars and carbohydrates are highly inflammatory. Keep in mind that there are many fad-like approaches to implementing the ketogenic diet. Research now indicates that is is better to practice a flexible ketogenic diet as long term ketosis can also contribute to insulin resistance over time.

The primary focus of a healthy ketogenic diet should be restoring metabolic flexibility through a focus on whole, organic foods, healthy fats, fish and pasture raised meats and non-starchy vegetables. The ketogenic diet is not a license to eat a pound of bacon for breakfast or a tub of sour cream and cheese for lunch. I recommend the bookKeto Flex by Ben Azadi if you are interested in exploring a ketogenic lifestyle in the healthiest way possible. Ben is especially knowledgeable about diabetes from his own personal story of losing his father to diabetes.
The Best Diet for Balancing Your Health
Diet is the biggest predictor for a healthy microbiome and balanced blood sugar. The Western diet, unfortunately, is not the optimal diet for your best health. The Western diet is high in processed foods, seed oils (canola and soybean), high fructose corn syrup, soft drinks, emulsifiers, preservatives and trans fats. You can change the bacteria in your gut in under thirty days by changing your diet . The best diet is one that is nutrient dense and includes lots of fresh, organic colorful vegetables, herbs and spices, organic blueberries, oily fish like salmon, pasture-raised meats and eggs, and incorporates oils like coconut and olive instead of the seed oils. A diet high in fresh vegetables and organic legumes (properly soaked and prepared) will increase the amount of fiber in your diet and reduce the simple carbohydrates. This will feed a population of healthy bacteria and reduce the growth of pathogens that prefer simple carbohydrates and sugar. A source of great help in lowering blood sugar is taking the right probiotic.
When I first formulated ourSugar Shift probiotic for my husband, John, my focus was on making mannitol in the gut because of research I had read about the ability of mannitol to stop the aggregation of the proteins. When I started to study mannitol chemistry, I learned how certain probiotic organisms can convert glucose and fructose into mannitol which humans don’t use but bacteria do. In a way, this mimics fasting to some degree because it converts the excess sugars in the upper GI tract. When I first made the product I was just thinking of mannitol production. I didn’t understand all of the research and implications of impaired glucose metabolism in Parkinson’s disease. Many of our customers now report that their blood glucose has improved and they no longer crave sugars. So many chronic diseases are fueled by sugar. Maybe we can help you makethe Shift. We start a clinical trial in diabetes and pre-diabetes beginning April 1, 2022. We are excited to see the results later this year.
With gratitude,

 Martha Carlin, is a “Citizen Scientist”,
systems thinker, wife of Parkinson’s warrior, John Carlin, and founder of The BioCollective , a microbiome company expanding
the reach of science and BiotiQuest, the first of it’s kind probiotic line. Since John’s diagnosis in 2002,
Martha began learning the science of agriculture, nutrition, environment, infectious disease, Parkinson’s
pathology and much more. In 2014, when the first research was published showing a connection between the gut
bacteria and the two phenotypes of Parkinson’s, Martha quit her former career as a business turnaround expert
and founded The BioCollective to accelerate the discovery of the impact of gut health on all human disease. Martha was a speaker at the White House 2016 Microbiome Initiative launch, challenging the scientific
community to “think in a broader context”. Her systems thinking background and experience has led to collaborations
across the scientific spectrum from neuroscience to engineering to infectious disease. She is a respected out of the
box problem solver in the microbiome field and brings a unique perspective to helping others understand the
connections from the soil to the food to our guts and our brains.
Martha Carlin, is a “Citizen Scientist”,
systems thinker, wife of Parkinson’s warrior, John Carlin, and founder of The BioCollective , a microbiome company expanding
the reach of science and BiotiQuest, the first of it’s kind probiotic line. Since John’s diagnosis in 2002,
Martha began learning the science of agriculture, nutrition, environment, infectious disease, Parkinson’s
pathology and much more. In 2014, when the first research was published showing a connection between the gut
bacteria and the two phenotypes of Parkinson’s, Martha quit her former career as a business turnaround expert
and founded The BioCollective to accelerate the discovery of the impact of gut health on all human disease. Martha was a speaker at the White House 2016 Microbiome Initiative launch, challenging the scientific
community to “think in a broader context”. Her systems thinking background and experience has led to collaborations
across the scientific spectrum from neuroscience to engineering to infectious disease. She is a respected out of the
box problem solver in the microbiome field and brings a unique perspective to helping others understand the
connections from the soil to the food to our guts and our brains.


Waking Up to a Probiotic Breakfast Can Do Wonders for Your Gut Health
Did you know that recent studies show people with poor gut diversity had lower quality of life? The health of your microbiome impacts your mental health, sleep, energy, the risk for chronic illnesses, and much more. A probiotic breakfast can...

Can Probiotic Supplements Make or Break Your Fast?
Intermittent fasting (IF) may have started as a fitness trend for weight loss, but today it's a go-to lifestyle choice for many. Practicing intermittent fasting has been linked with health benefits such as lowering blood sugar and insulin, preventing heart...













 Martha Carlin
Martha Carlin






